Dr. Sarah Cavanaugh discusses biomedical research in her talk, "Homo sapiens: the ideal animal model"
Biology and preclinical medicine rely heavily upon research
in animal models such as rodents, dogs, and chimps. But how translatable are
the findings from these animal models to humans? And what alternative systems
are being developed to provide more applicable results while reducing the number
of research animals?
 |
| Image courtesy of PCRM |
Last Thursday, PSPG invited Dr. Sarah Cavanaugh from the
Physicians Committee for Responsible Medicine to discuss these issues. In her
talk entitled, “Homo sapiens: the ideal animal model,” she emphasized that we
are not particularly good at translating results from animal models into human
patients. Data from the FDA says that 90% of drugs that perform well in animal
studies fail when tested in clinical trials.
It may seem obvious, but it is important
to point out that the biology of mice is not identical to human biology.
Scientific publications have demonstrated important dissimilarities in regards
to the pathology of inflammation,
diabetes,
cancer, Alzheimer’s,
and heart disease.
All scientists understand that model systems have
limitations, yet they have played an integral role in shaping our understanding
of biology. But is it possible to avoid using experimental models entirely and
just study human biology?
The ethics of studying biology in people are different from those of studying biology in animals. The “do no
harm” code of medical ethics dictates that we can’t perform experiments that
have no conceivable benefit for the patient, so unnecessarily invasive
procedures can not be undertaken just to obtain data. This limitation restricts
the relative amount of information we can obtain about human biology as
compared to animal biology. Regardless,
medical researchers do uncover important findings from human populations. Dr.
Cavanaugh points out that studies of risk factors (both genetic and
environmental) and biomarkers are important for understanding diseases, and
non-invasive brain-imaging has increased our understanding of neurodegenerative
diseases like Alzheimer’s.
Yet these are all correlative measures. They show that
factor X correlates with a higher risk of a certain disease. But in order to
develop effective therapies, we need to understand cause and effect
relationships - in other words, the mechanism. To uncover mechanisms researchers need to be
able to perturb the system and measure physiological changes or observe how a
disease progresses. Performing these studies in humans is often hard,
impossible, or unethical. For that reason, researchers turn to model systems in
order to properly control experimental variables to understand biological
mechanisms. We have learned a great deal about biology from animal models, but
moving forward, can we develop models that better reflect human biology and
pathology?
Using human post-mortem samples and stem cell lines is one
way to avoid species differences between animals and humans, but studying
isolated cells in culture does not reflect the complex systems-level biology of
a living organism. To tackle this problem, researchers have started designing ways to
model 3D human organs in vitro, such as the brain-on-a-chip system.
Researchers also have envisioned using chips to model a functioning body using
10 interconnected tissues representing organs such as the heart, lungs, skin,
kidneys, and liver.
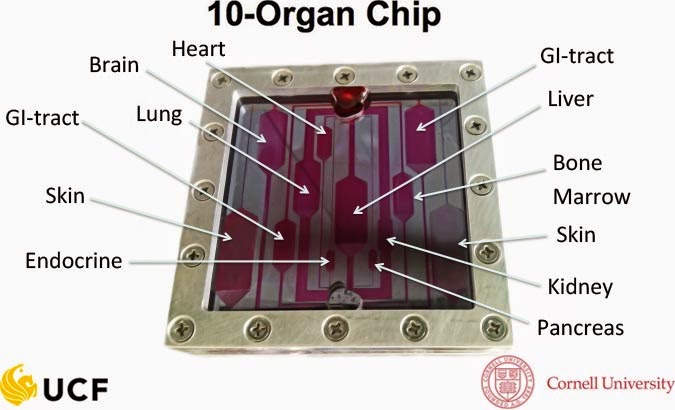 |
| Image from: http://nanoscience.ucf.edu/hickman/bodyonachip.php |
Dr. Cavanaugh explained that toxicology is currently a field
where chip-based screening shows promise. It makes sense that organs-on-a-chip
technology could be useful for screening drug compounds before testing in
animals. Chip-screening could filter out many molecules with toxic effects,
thus reducing the number of compounds that are tested in animals before being
investigated clinically.
A major counterpoint raised during the discussion was
whether replacing animal models with human organs on a chip was simply
replacing one imperfect, contrived model with another. Every model has
limitations, so outside of directly testing therapeutics in humans, it is
unlikely that we will be able to create a system that perfectly reflects the
biological response in patients. The question then becomes, which models are
more accurate? While ample data shows the limitations of animal models, very
little is available showing that alternatives to animal-free models perform
better than existing animal models. Dr Cavanaugh argues, however, that there is
an opportunity to develop these models instead of continuing to pursue research
in flawed animal models. “I
don’t advocate that we end all animal research right now, rather that we invest
in finding alternatives to replace the use of animals with technologies that
are more relevant to human biology.”
This topic can ignite a passionate debate within the medical
research community. Animal models are the status quo in research, and they are
the gatekeepers in bench-to-bedside translation of scientific discoveries into
therapeutics. In the absence of any shift in ethical standards for research,
replacing animal models with alternatives will require mountains of strong data
demonstrating better predictive performance. The incentives exist, though. Drug companies spend roughly $2.6 billion to gain market approval for a new
prescription drug.
Taking a drug into human trials and watching it fail is a huge waste of money.
If researchers could develop new models for testing drugs that were more
reliable than animal models at predicting efficacy in humans, it’s safe to say that Big
Pharma would be interested. Very interested.
-Mike Allegrezza
 |
| "Wistar rat" by Janet Stephens via Wikimedia Commons |


Comments
Post a Comment